Any drug or molecule is granted 20 years of patent protection, after expiration of which revenue for patented drug decreases sharply as generic substitutes enter the market. The point at which patent protection expires is known as the patent cliff. In such scenarios, drug manufacturers may employ various life-cycle management patent strategies. To extend the exclusivity of the patented product the drug manufacturers may develop variations of originator products and filing patents on isomers, metabolites, prodrugs, new drug formulations (e.g., extended-release versions), and fixed-dose combinations. Amid a slew of drugs going off patent or expiring in 2023 is Swiss drugmaker Novartis’ heart failure blockbuster drug Vymada or Entresto® approved for treatment of symptomatic chronic heart failure with reduced and preserved ejection fraction.
The journey of the blockbuster drug
Entresto® belongs to a class of drugs called angiotensin receptor-neprilysin inhibitors (ARNIs). It was first approved in July 2015 for patients with Heart Failure with Reduced Ejection Fraction (HFrEF). Later, in February 2021, Entresto’s indication was expanded by FDA to also include patients with Heart Failure with Preserved Ejection Fraction (HFpEF). The February 2021 supplement approval by FDA gave Entresto® a three-year exclusivity.
Globally, Entresto® is estimated to have a market size of $4 billion. According to a report published in September 2022, by Fierce Pharma here, at least 18 drugmakers have potential generic versions of Entresto® and plan to enter market in July 2023 when a regulatory stay that prevents the FDA from granting final generic approvals lifts.
In India, Entresto is sold as Vymada by Novartis and the product’s secondary brands Cidmus and Azmarda are marketed by local partners Lupin Limited and Cipla Limited, respectively. Vyamada is one of the fastest growing drugs with a cardiac market of approximately Rs 23,000 Crore in India alone. Numerous Indian drug makers are in a tussle to launch the generic version of this heart failure medicine. In 2019, Novartis AG had raised a dispute, alleging that the Indian firms were attempting to infringe the patent for its pharmaceutical composition valsartan and sacubitril as a sodium salt complex. The Delhi High Court had then passed an injunction in favour of Novartis AG against four Indian generic manufacturers i.e., Natco Pharma, Torrent Pharmaceuticals, Eris LifeSciences and Windlas Biotech, in a patent infringement suit.
Timeline of patents related to Entresto®
The base patent of Entresto® i.e., the original patent (US7468390B2), was filed in January 2003 by Novartis claiming the pharmaceutical composition having active ingredient as a combination of Valsartan and Sacubitril achieving a greater anti-hypertensive effect when administered in combination than the sum of Valsartan and Sacubitril administered alone. Sacubitril belongs to a class of drugs called neprilysin inhibitors and valsartan belongs to a class of drugs called angiotensin receptor blockers (ARBs). The patent also claimed a kit comprising a single packaging pharmaceutical composition with separate containers for Valsartan and Sacubitril composition. This patent has an adjusted expiration date on 23rd November 2023. However, the same application filed in India (IN229051) by Novartis AG, is due to expire on 14th January 2023.
In addition, Novartis sought numerous additional patents on Entresto®, which are enlisted in the Orange Book. These secondary patents have further extended the exclusivity of the drug till 2036 by protecting incremental changes such as methods of treatment, dosage regimens, etc. However, secondary patents have a higher chance of being challenged by generic manufacturers and overturned in court than patents for the active ingredient.
In this post, we would be discussing the chronology and scope of secondary patents filed by Novartis with respect to Entresto®.
Three years after the base patent, Novartis filed a secondary patent (US8877938B2) in November 2006, covering dual-acting single molecule having Valsartan and Sacubitril linked via non-covalent bonding, or supramolecular complexes, or linked pro-drugs, such as mixed salts or co-crystals. This patent provides patent protection for Entresto® till 2027—more than 4 years beyond the expiration of the Entresto® molecule base patent.
Subsequently, another patent US8101659B2 was filed by Novartis in June 2008, additionally covering a pharmaceutically acceptable carrier along with the active ingredients and administering the composition as a combination of Valsartan and Sacubitril, in a 1:1 ratio in form of a capsule or tablet. The patent also extended the scope of the composition for the treatment of hypertension or heart failure which was not claimed in the base patent. The patent was anticipated for expiry on 14th January 2023; however, it was granted a Period of Extension for 732 days on the grounds of time lost while awaiting premarket government approval from FDA. This patent now has an adjusted expiration date of 15th January 2025.
Along with the base patent, two secondary patents are also set to expire on 14th January 2023. These are, US8404744B2 filed on December 2011, additionally defining the salt form of Sacubitril in the composition, and US8796331B2 filed on November 2012 as a “method-of-use” patent covering a method for the treatment of hypertension and heart failure, by administering to a patient a therapeutically effective amount of the Valsartan and Sacubitril.
US9517226B2 and US9937143B2 further expanded Entresto’s indication by including patients with Heart Failure with Preserved Ejection Fraction. The patents were filed by Novartis in August 2013 and November 2016 respectively. Both the patents are “method-of -use” patents covering administering supramolecular complex of Valsartan and Sacubitril (LCZ696) for heart failure especially the treatment of atrial fibrillation or for delaying the time until the new onset of atrial fibrillation. The methods also covered a dosing regimen wherein LCZ696 is administered daily twice at a dose of 50 mg, 100 mg, 200 mg, or 400 mg. These patents thus cover protection for Entresto® till 2033—10 years beyond the expiration of the Entresto® molecule base patent.
Another “method-of-use” patent i.e., US9388134B2 was filed by Novartis in June 2014 expanding the use of LCZ696 for chronic heart failure and hypertension. The characterization of LCZ696 was also specifically disclosed in this patent. The patent is set to expire in November 2026.
Filing secondary patents claiming dosage regimen, is one of the ways to extend the market exclusivity of an approved drug beyond the lifetime of the patent. In May 2016, Novartis filed a secondary patent (US11058667B2) claiming a dosage regimen for Entresto® for treating chronic heart failure with reduced ejection fraction. As per the claims, the patients are administered a twice-daily target dose of 200 mg of sacubitril and valsartan combination corresponding to 97 mg of sacubitril and 103 mg of valsartan. The patent also claims a dosage regimen wherein the drug titration process includes a twice-daily starting dose of 50 mg of sacubitril and valsartan, for 3 to 4 weeks, followed by a twice-daily dose of 100 mg of sacubitril and valsartan, for 3 to 4 weeks followed by the twice daily target dose of 200 mg thereafter. The dosage regimen also includes the 50 mg twice daily dose for patients taking a low dose of an angiotensin-converting enzyme (ACE) inhibitor or a low dose of an angiotensin II receptor blocker (ARB) (or a low dose equivalent of <10 mg of enalapril per day) before initiating treatment with sacubitril and valsartan. The patent is set to expire in May 2036.
The indication for Entresto® was further extended to a treatment method for patients having heart failure with preserved ejection fraction (HF-PEF) in US11135192B2 filed by Novartis in June 2018. The patent disclosed a dosage regimen wherein Valsartan and Sacubitril combination can be administered as 50 mg, 100 mg, or 200 mg dose daily twice for at least 36 weeks. The patent is set to expire in August 2033.
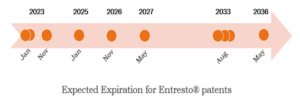
The following Figure shows the timeline of expected expiration for patents filed by Novartis with respect to Entresto®.
Conclusion
In conclusion, although Entresto’s base patent along with a few other secondary patents are anticipated to expire on or before 14th January 2023, several patents including the method-of-use patents, and patents covering dosage regimen are yet to expire and these will provide the rudimentary exclusive right to Entresto® till 2036.
Having said that, in India, the situation is a bit different. Owing to the famous Section 3(d) for non-patentable inventions, new use or a new form of a known substance is excluded from patentability. It is therefore likely that these improvement/secondary patents were not granted or pursued in India in the first place. Therefore, within the jurisdiction of India, most rights over the drug combination may be considered expired opening the gates for the generic market to take over the manufacturing and commercialization of the product.
Dr. Vasundhara Paliwal and Sushma H.A., Associate Patents at BananaIP Counsels, have compiled the following data. Please note that this data has been sourced from both primary and secondary sources and may not have been verified by BananaIP‘s reporters. If you would like to make any corrections or take down any of the information published in this bulletin, please contact contact@bananaip.com.



